Corrosion results from chemical reactions that naturally occur externally and internally in heat exchangers. Corrosion happens when there is a disturbance in the flow of water, which contains suspended solid particles and chemical compositions rich in chlorides. Results in a loss of stability of the protective cuprous oxide layer. Without this layer, the heat exchanger has no protection from erosion and fouling.



Types of corrosions:
- Pitting Corrosion- Formation of holes or pits on the surface
- Stress Corrosion- Fractures of surfaces by residual stress and environment
- Gas Corrosion
- Salt Corrosion
Corrosive Environments:
- Coastal/Marine
- Industrial
- Combination Marine/Industrial
- Urban
- Rural
Results of corrosion:
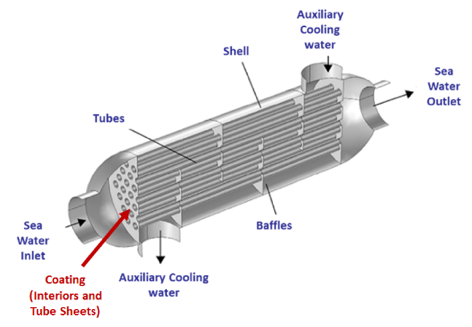
Current Approaches
The most commonly used method to prevent corrosion is through water monitoring, or by paying close attention to the substances that are used in accordance to the heat exchanger’s makeup. There are various coatings and epoxies; however, long-lasting prevention has not been fully accomplished due to issues with durability, an increased heat transfer coefficient, and cost. Adding zinc anodes to the heat exchanger bonnet has also been used, but due to the overwhelming amount of metal lost, zinc anodes need to be inspected regularly and replaced as required.
HeatX has been demonstrated to significantly reduce the frequency of costly maintenance operations, and extend the overall “useful life” of assets. HeatX has been qualified to show corrosion resistance, abrasion resistance, and chemical compatibility with both water, hydrocarbon, and mixed phase conditions.
The water based formulation can be flexibly applied to unprepared corroded surfaces and still achieve full performance. In addition, the overall thickness of the HeatX coating is less than 4 mil, and does not affect the overall heat transfer performance.
Benefits of HeatX:
Recent Comments